 Rheumatoid arthritis often starts in young adults and flares up in middle age. The condition is more common in women than in men. The causes of rheumatoid arthritis are complex and not perfectly understood.
Rheumatoid arthritis often starts in young adults and flares up in middle age. The condition is more common in women than in men. The causes of rheumatoid arthritis are complex and not perfectly understood.Rheumatoid arthritis (RA) is an inflammatory autoimmune disease that is characterized by the destruction of joint cartilage and inflammation of the synovium (joint fluid) (Abbas et al., 1994). The joints of the extremities, particularly the metacarpophalangeal, interphalangeal, and wrist joints, are the primary diseased sites, but as the disease progresses, the larger joints, especially the knees, can also be affected. The disease is an inflammatory arthritis that involves stiffness, pain, swelling, and erythema. As the disease progresses, osteoporosis of the contiguous bone, destruction of joint cartilage, bone resorption, and displacement by ankylosis occur. Subcutaneous nodules may also form at pressure points, particularly on the anterior side of the forearms, in 15-20% of reported cases. Systemic complications include damage to various organs (lungs, pleura, pericardium, myocardium, eyes, and central nervous system) due to inflammatory reactions. The outcome of the disease varies among patients, ranging from complete recovery to total incapacitation within years of the onset of the disease. In the most severe cases, vasculitis may occur. Women represent 75% of the reported cases of RA. There are two major peaks of incidence with regard to age: one between 30 and 40 years and one between 50 and 60 years (Bach, 1982).
Early experimental work focused on the importance of B-cells in the pathology of RA. Later interest has focused on T-cells. Observations that RA is strongly associated with certain MHC (major histocompatibility complex) alleles and that activated T-cells are found in affected joints have indicated that much focus should be given to cell-mediated immune responses. Whether activated T-cells initially respond to a microbial antigen, superantigen, or self-constituent is unclear to date (Chini et al., 2002).

Immunology and the Causes of RA
The development of lesions in rheumatoid arthritis appears to include both cell-mediated and humoral responses. Early research aimed at identifying the cells present in the inflamed synovium, and it has been concluded that CD4+ T-cells, activated B-lymphocytes, and plasma cells, as well as well-formed lymphoid follicles with germinal centers (in more severe cases), are present in the synovium of patients diagnosed with RA (Abbas et al., 1994). T-cells are the dominant cell type in the synovial filtrate of patients with RA, and there is at least a partial therapeutic effect of T-cell depletion for these patients (Berner et al., 2000). Current understanding of RA suggests that TH1 cells that are specific for a particular antigen (which has yet to be identified) are present in the joints of people with RA. This antigen activates T-cells to release lymphokines that cause local inflammation at the joints. The clinical manifestations of this inflammation include swelling, accumulation of polymorphonuclear leukocytes and macrophages at the site of inflammation, cartilage damage, and, thus, destruction of the joint (Janeway et al., 2001).
T-cells are the dominant type of cell that infiltrates the synovial membrane in RA. In many patients, the lymphocytes that infiltrate the tissues are organized into follicles that are structurally similar to germinal centers, which strongly supports the idea that RA is an antigen-driven immune response. Recent studies, however, have focused on the possibility that T-cells have an alternative role besides antigen recognition in the joints. Irregularities are found in the global and synovial T-cell receptor (TCR) repertoire of RA patients. The T-cell repertoire shows an emergence of clonal T-cell populations. CD4 T-cell clones are present uniformly in circulation and infiltrate into synovial lesions. CD8 T-cells clones are also found in patients with RA; although these clones are not limited to RA patients, they are larger and more frequent in people with the disease. CD4 T-cells are autoreactive to ubiquitous antigen, do not express the CD28 molecule, and do not require costimulatory signals to secrete cytokines. A study performed at the Mayo Clinic in 2002 found that RA is associated with a generalized defect in diversity maintenance of the TCR repertoire. This results in clonal expression of peripheral T-cells and repertoire contraction. Of significance, the researchers found that these irregularities involve memory and naïve T-cells, which suggests that the abnormality is a defect in T-cell homeostasis, rather than a consequence of antigen-recognition in the synovium. Specifically, the study showed that the CD4 TCR beta-chain diversity is limited in RA patients. Loss in diversity leads to the insufficient influx of ‘novel’ T-cells. Peripheral T-cells respond by replicating vigorously to compensate for the loss of new production, and clonal populations are formed. The multiclonal T-cells proliferation and the associated repertoire contraction have important implications for the course of the disease. It appears that the T-cell clones recognize a ubiquitously distributed autoantigen in RA patients. A recent study by Kouskoff et al. describes that transgenic mice expressing a TCR that recognizes self-MHC molecules develops arthritis that resembles RA but no other autoimmune diseases. To date, however, the autoantigen remains to be elucidated (Wagner et al., 1998).
Unusual distortions of the naïve T-cell repertoire have also been examined. These defects have led researchers to conclude that abnormal T-cell development and differentiation occurs in RA; autoimmunity could be caused by the inappropriate development and maturation of T-cells. RA patients appear to have fewer naïve T-cells with atypical phenotypes as compared to controls; these data suggest abnormal T-cell proliferation and phenotypic differentiation occur in response to inflammatory stimuli in patients with RA. These atypical cells appear to have a reduced threshold for activation and may bypass lymph nodes in favor of peripheral sites, which, during an infection, can lead to autoreactivity (Ponchel et al., 2002). Following this line of thought, hyperfunctioning T-cells could secrete local mediators in the synovial fluid, leading to arthritis. This hypothesis is supported by the fact that numerous T cells are found in the synovium of affected individuals. Also, injection of T-cell culture supernatants can induce inflammatory arthritis that resembles the clinical manifestations of RA. In this scenario, immune complex formation may be caused by T-cell hyperfunction through a helper effect, and the immune complexes would cause certain symptoms, including nodules and vasculitis (Bach, 1982).
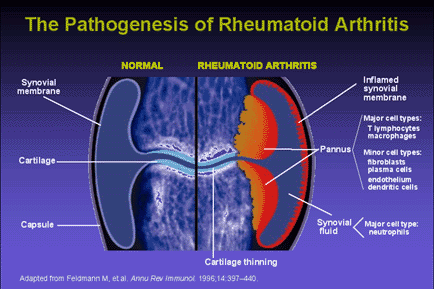
Fig. 2 The Pathogenesis of Rheumatoid Arthritis. The development of lesions in rheumatoid arthritis appears to include both cell-mediated and humoral responses. CD4+ T-cells, activated B-lymphocytes, and plasma cells, as well as well-formed lymphoid follicles with germinal centers (in more severe cases), are present in the synovium of patients diagnosed with RA. T-cells are the dominant cell type in the synovial filtrate of patients with RA. Cytokine secretion by activated T-cells leads to an inflamed synovium and the formation of pannus (Abbas et al., 1994).

Treatments
Anti-TNF-alpha Treatments
Cytokines are protein mediators that are implicated in nearly all biological processes, including cell growth, differentiation, inflammation, and immunity. In patients with RA, nearly all cytokines are expressed in RA tissue in a continuous fashion. Researchers previously thought that targeting the blockade of a particular cytokine would be an ineffective therapeutic treatment since, they supposed, other cytokines would take over the role of the blocked cytokine. Research with RA has led them to a different hypothesis. IL-1 has been shown to induce the destruction of cartilage and bone, and five signals present in the RA synovium (IFN-gamma, GM-CSF, TNF-alpha, immune complexes, and IL-1 itself) help to regulate IL-1 production. However, a study done at the Kennedy Institute of Rheumatology found that blocking TNF-alpha abolished IL-1 bioactivity. Anti-TNF antibodies were also found to downregulate GM-CSF, IL-6, and IL-8. Researchers determined that pro-inflammatory cytokines are co-regulated, and the key inflammatory cytokines are TNF-alpha and IL-2. This led the way for the development of anti-TNF-alpha treatments; both soluble-receptor antagonists and cytokine antibodies have been developed as anti-TNF-alpha therapies (Feldmann et al., 1999).
Medications Commonly Used to Treat Rheumatoid Arthritis (Rang et al., 1995)
Four types are medications are commonly used to treat rheumatoid arthritis. These include aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs), disease-modifying antirheumatic drugs (DMARDs), immuno-suppressants, and corticosteroids (glucocorticoids).
NSAIDs have three major types of effect. They are anti-inflammatory agents; they have an analgesic effect (reduction of pain); and they have an antipyretic effect (lowering of raised temperature). Examples of these drugs include, but are not limited to, plain aspirin, buffered aspirin, ibuprofen (Advil ®, Motrin IB ®), ketoprofen (Orudis®), naproxen (Naprosyn®), celecoxib (Celebrex®), and rofecoxiv (Vioxx®).
Anti-inflammatory effects: The primary action of the drugs is to inhibit arachidonate cyclo-oxygenase and, thus, to inhibit the production of prostaglandins and thromboxanes. One type of cyclo-oxygenase, COX-2, is induced in activated inflammatory cells and is the enzyme that produces the prostanoid inflammatory mediators. NSAIDs reduce the components of inflammation that are caused by COX-2 action, which include vasodilation, edema, and pain. These drugs have no effect on the processes that contribute to tissue destruction in RA; they simply reduce the generation of toxic O2 products and inhibit lymphocyte activation.
Antipyretic effects: NSAIDs inhibit prostaglandin production in the hypothalamus. During an inflammatory reaction, macrophages release IL-1, which stimulates the generation of E-type prostaglandins in the hypothalamus, which elevate the body temperature of the individual. NSAIDs, thus, act to reduce body temperature when it is raised above normal levels.
Analgesic effects: NSAIDs are effective against pain that is caused by prostaglandins acting on nociceptors (ie. pain associated with inflammation or tissue damage). Decreased prostaglandin production leads to less sensitization of nociceptic nerve endings to the inflammatory mediators, bradykinin and 5-hydroxytryptamine (Rang et al., 1995).
DMARDs are distinct from NSAIDs in that they do more than simply alleviate the symptoms of RA. They are used to alter the course of the disease and prevent joint and cartilage destruction. Three types of DMARDs are widely used; these include gold compounds (Myochrysine®, Ridaura®), penicillamine (Cuprimine®, Depen®), and chloroquine (Plaquenil®). Although their mechanisms of action have not been fully elucidated, their effects have had a profound effect on RA patients.
Gold Compounds: Gold compounds help to stop the progression of bone and joint damage in RA. Pain and joint swelling are reduced when these drugs help to reduce the concentration of rheumatoid factor. Although the exact mechanism of action is not fully understood, any or all of the following effects can contribute to the mechanism: inhibition of mitogen-induced lymphocyte proliferation, reduction of lysosomal enzymes, reduction in the production of toxic O2 metabolites from phagocytes, inhibition of chemotaxis of neutrophils, and reduction in IL-2 production.
Penicillamine: Penicillamine prevents the maturation of newly synthesized collagen. Although the mechanism of action is not fully understood, it has been observed that joint swelling subsides, nodules disappear, and IL-1 production is reduced. About 75% of patients with RA respond to this treatment.
Chloroquine: Anti-malarial drugs cause remission of RA but do not stop bone destruction. They inhibit mitogen-induced lymphocyte proliferation and decrease leukocyte chemotaxis. They also reduce IL-1 production (Rang et al., 1995)
Immuno-suppressants, which are considered DMARDs, restrain an overly-active immune system. These drugs include cyclosporine (Sandimmune®, Neoral®) and cytotoxic agents such as azathioprene (Imuran®).
Cyclosporine: Cyclosporine suppresses both cell-mediated and humoral responses. It acts on T-lymphocytes at the induction stage to stop clonal proliferation. The transduction pathway for lymphokine synthesis is inhibited, mainly the production of IL-2. It also inhibits IL-2 receptor expression on T-cells that respond to IL-2. The induction of cytotoxic T-cells is also inhibited. B-cell responses are suppressed because lymphokine synthesis and secretion from activated T-cells is inhibited.
Azathioprine: Azathioprine is metabolized to give mercaptiopurine, which is a purine analogue that inhibits DNA synthesis. This drug depresses both cell-mediated and humoral responses since it inhibits clonal proliferation by a cytotoxic action on dividing cells (Rang et al., 1995).
Corticosteroids have anti-inflammatory and immunosuppressive effects. They include prednisone (Deltasone®, Orasone®), hydrocortisone, dexamethasone, and methylprednisolone (Medrol®). These drugs reduce vasodilation and decrease fluid exudation. In areas of acute inflammation, they decrease the number and activity of leukocytes (decreased action of T-helper cells and reduced clonal proliferation of T-cells due to decreased production of IL-2; decreased release of monocytes and increased number of neutrophils from the bone marrow). In areas of chronic inflammation, the activity of mononuclear cells is decreased. In lymphoid areas, there is decreased clonal expansion of T- and B-cells and decreased action of cytokine-secreting T-cells. These drugs decrease the production of many cytokines, including IL-1, IL-2, IL-3, IL-4, IL-5, Il-6, IL-8, TNF-alpha, and GM-CSF. They also help to decrease the complement component in the blood. Overall, they reduce chronic inflammation and autoimmune reactions. Glucocorticoids act by interacting with intracellular receptors; the steroid-receptor complex interacts with DNA to modify gene transcription. These drugs perform their anti-inflammatory and immunosuppressive actions as follows: inhibition of transcription of the gene for COX-2; blockage of vitamin-D3-mediated induction of the osteocalcin gene in osteoclasts and modification of the transcription of the collagenase gene; and increased synthesis of an anti-inflammatory mediator protein (lipocortin 1) which inhibits phospholipase A2 and blocks the production of platelet-activating factor (Rang et al., 1995).
|
Bookmark this post:
|
|

0 comments
Post a Comment